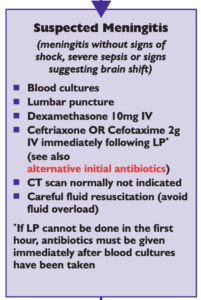
Many local and national guidelines for the management of anaphylaxis exist, but did you know there was a World Allergy Organization, and it has a very detailed guideline on this important life threatening condition?

Some interesting snippets from the guideline are included here
Anaphylaxis and cardiac disease
- Anaphylaxis can precipitate acute myocardial infarction in susceptible individuals: in patients with ischemic heart disease, the number and density of cardiac mast cells is increased, including in the atherosclerotic plaques. Mediators released during anaphylaxis contribute to vasoconstriction and coronary artery spasm.
- Epinephrine is not contraindicated in the treatment of anaphylaxis in patients with known or suspected cardiovascular disease, or in middle-aged or elderly patients without any history of coronary artery disease who are at increased risk of ACS only because of their age. Through its beta-1 adrenergic effects, epinephrine actually increases coronary artery blood flow because of an increase in myocardial contractility and in the duration of diastole relative to systole.
- Glucagon has noncatecholamine-dependent inotropic and chronotropic cardiac effects, and is sometimes needed in patients taking a beta-adrenergic blocker who have hypotension and bradycardia and who do not respond optimally to epinephrine.
- Anticholinergic agents are sometimes needed in beta-blocked patients, for example, atropine in those with persistent bradycardia or ipratropium in those with epinephrine-resistant bronchospasm.
How quickly can untreated anaphylaxis kill you?
Studies show median times to cardiorespiratory arrest after exposure to the offending stimulus were 5 minutes after administration of contrast media or drugs, 15 minutes after an insect sting, and 30 minutes after food ingestion.
What about confirming the diagnosis with serum tryptase measurements?
- Blood samples for measurement of tryptase levels are optimally obtained 15 minutes to 3 hours after symptom onset.
- Blood samples for measurement of histamine levels are optimally obtained 15–60 minutes after symptom onset. These tests are not specific for anaphylaxis.
- Increased serum tryptase levels are often found in patients with anaphylaxis from insect stings or injected medications, and in those who are hypotensive
- However, levels are often within normal limits in patients with anaphylaxis triggered by food and in those who are normotensive
- Serial measurement of tryptase levels during an anaphylactic episode, and measurement of a baseline level after recovery are reported to be more useful than measurement at only one point in time.
- Normal levels of either tryptase or histamine do not rule out the clinical diagnosis of anaphylaxis
How does epinephrine help?
- Epinephrine is life-saving because of its alpha-1 adrenergic vasoconstrictor effects in most body organ systems (skeletal muscle is an important exception) and its ability to prevent and relieve airway obstruction caused by mucosal edema, and to prevent and relieve hypotension and shock.
- Other relevant properties in anaphylaxis include its beta-1 adrenergic agonist inotropic and chronotropic properties leading to an increase in the force and rate of cardiac contractions, and its beta-2 adrenergic agonist properties such as decreased mediator release, bronchodilation and relief of urticaria
- Epinephrine in a dose of 0.01 mg/kg of a 1:1,000 (1 mg/mL) solution injected promptly by the intramuscular route is effective and safe in the initial treatment of anaphylaxis. In other anaphylaxis scenarios, this low first-aid dose is unlikely to be effective. For example, if shock is imminent or has already developed, epinephrine needs to be given by slow intravenous infusion, ideally with the dose titrated according to noninvasive continuous cardiac monitoring.
What is the empty ventricle syndrome?
- Patients with anaphylaxis should not suddenly sit, stand, or be placed in the upright position.
- Instead, they should be placed on the back with their lower extremities elevated or, if they are experiencing respiratory distress or vomiting, they should be placed in a position of comfort with their lower extremities elevated.
- This accomplishes 2 therapeutic goals: 1) preservation of fluid in the circulation (the central vascular compartment), an important step in managing distributive shock; and 2) prevention of the empty vena cava/empty ventricle syndrome, which can occur within seconds when patients with anaphylaxis suddenly assume or are placed in an upright position.
- Patients with this syndrome are at high risk for sudden death. They are unlikely to respond to epinephrine regardless of route of administration, because it does not reach the heart and therefore cannot be circulated throughout the body
Should we give antihistamines, beta 2 agonists, and steroids?
The evidence base for use of these second line medications in the initial management of anaphylaxis, is extrapolated mainly from their use in treatment of other diseases such as urticaria (antihistamines) or acute asthma (beta-2 adrenergic agonists and glucocorticoids). Concerns have been raised that administering one or more second-line medications potentially delays prompt injection of epinephrine, the first-line treatment
Is ‘biphasic anaphylaxis’ a real phenomenon we should be concerned about?
- Biphasic anaphylaxis occurs when symptoms recur within 1–72 hours (usually within 8–10 hours) after the initial symptoms have resolved, despite no further exposure to the trigger.
- It occurs in up to 23% of adults and up to 11% of children.
- After apparent resolution of symptoms, duration of monitoring in a medically supervised setting should be individualized. For example, patients with moderate respiratory or cardiovascular compromise should be monitored for at least 4 hours, and if indicated, for 8–10 hours or longer.
- Protracted uniphasic anaphylaxis is uncommon, but can last for days.
World Allergy Organization Guidelines for the Assessment and Management of Anaphylaxis
World Allergy Organization Journal 2011;4(2):13-37 Full Text
[EXPAND click for abstract]
The illustrated World Allergy Organization (WAO) Anaphylaxis Guidelines were created in response to absence of global guidelines for anaphylaxis. Uniquely, before they were developed, lack of worldwide availability of essentials for the diagnosis and treatment of anaphylaxis was documented. They incorporate contributions from more than 100 allergy/immunology specialists on 6 continents. Recommendations are based on the best evidence available, supported by references published to the end of December 2010. The Guidelines review patient risk factors for severe or fatal anaphylaxis, co-factors that amplify anaphylaxis, and anaphylaxis in vulnerable patients, including pregnant women, infants, the elderly, and those with cardiovascular disease. They focus on the supreme importance of making a prompt clinical diagnosis and on the basic initial treatment that is urgently needed and should be possible even in a low resource environment. This involves having a written emergency protocol and rehearsing it regularly; then, as soon as anaphylaxis is diagnosed, promptly and simultaneously calling for help, injecting epinephrine (adrenaline) intramuscularly, and placing the patient on the back or in a position of comfort with the lower extremities elevated. When indicated, additional critically important steps include administering supplemental oxygen and maintaining the airway, establishing intravenous access and giving fluid resuscitation, and initiating cardiopulmonary resuscitation with continuous chest compressions. Vital signs and cardiorespiratory status should be monitored frequently and regularly (preferably, continuously). The Guidelines briefly review management of anaphylaxis refractory to basic initial treatment. They also emphasize preparation of the patient for self-treatment of anaphylaxis recurrences in the community, confirmation of anaphylaxis triggers, and prevention of recurrences through trigger avoidance and immunomodulation. Novel strategies for dissemination and implementation are summarized. A global agenda for anaphylaxis research is proposed.
[/EXPAND]
This month has also seen the publication guidelines from the UK’s National Institute for Health & Clinical Excellence, entitled ‘Anaphylaxis: assessment to confirm an anaphylactic episode and the decision to refer after emergency treatment for a suspected anaphylactic episode’
Their guideline summary is as follows:
After a suspected anaphylactic reaction in adults or young people aged 16 years or older, take timed blood samples for mast cell tryptase testing as follows:
- a sample as soon as possible after emergency treatment has started
- a second sample ideally within 1–2 hours (but no later than 4 hours) from the onset of symptoms.
After a suspected anaphylactic reaction in children younger than 16 years, consider taking blood samples for mast cell tryptase testing as follows if the cause is thought to be venom-related, drug-related or idiopathic:
- a sample as soon as possible after emergency treatment has started
- a second sample ideally within 1–2 hours (but no later than 4 hours) from the onset of symptoms.
Patients who have had emergency treatment for suspected anaphylaxis should be observed for 6–12 hours from the onset of symptoms, depending on their response to emergency treatment
After emergency treatment for suspected anaphylaxis, offer people a referral to a specialist allergy service (age-appropriate where possible) consisting of healthcare professionals with the skills and competencies necessary to accurately investigate, diagnose, monitor and provide ongoing management of, and patient education about, suspected anaphylaxis.
After emergency treatment for suspected anaphylaxis, offer people (or, as appropriate, their parent and/or carer) an appropriate adrenaline injector as an interim measure before the specialist allergy service appointment.
Before discharge a healthcare professional with the appropriate skills and competencies should offer people (or, as appropriate, their parent and/or carer) the following:
- information about anaphylaxis, including the signs and symptoms of an anaphylactic reaction
- information about the risk of a biphasic reaction
- information on what to do if an anaphylactic reaction occurs (use the adrenaline injector and call emergency services)
Anaphylaxis: assessment to confirm an anaphylactic episode and the decision to refer after emergency treatment for a suspected anaphylactic episode
CG134 Anaphylaxis: NICE guideline