 I discuss the bad old days of poor training and lack of supervision with emergency physician and retrieval specialist Dr Karel Habig.
I discuss the bad old days of poor training and lack of supervision with emergency physician and retrieval specialist Dr Karel Habig.
The skeletons are coming out of the closet!
Resus.ME! June 2012
Monthly Archives: June 2012
Confidential stuff – in hospital cardiac arrests
 A new report describes room for improvement in the care of cardiac arrest patients in hospital1.
A new report describes room for improvement in the care of cardiac arrest patients in hospital1.
The National Confidential Enquiry into Patient Outcome and Death (NCEPOD) aimed to describe variability and identify remediable factors in the process of care of adult patients who receive resuscitation in hospital, including factors which may affect the decision to initiate the resuscitation attempt, the outcome and the quality of care following the resuscitation attempt, and antecedents in the preceding 48 hours that may have offered opportunities for intervention to prevent cardiac arrest.
Data were captured over a 14 day study period in late 2010 from UK hospitals, and were reviewed by an expert panel.
The summary is available here. I have picked out some findings of interest:
- An adequate history was not recorded in 70/489 cases (14%) and clinical examination was incomplete at first contact in 117/479 cases (24%).
- Appreciation of the severity of the situation was lacking in 74/416 (18%).
- Timely escalation to more senior doctors was lacking in 61/347 (18%).
- Decisions about CPR status were documented in the admission notes in 44/435 cases (10%). This is despite the high incidence of chronic disease and almost one in four cases being expected to be rapidly fatal on admission.
- Where time to first consultant review could be identified it was more than 12 hours in 95/198 cases (48%).
- Appreciation of urgency, supervision of junior doctors and the seeking of advice from senior doctors were rated ‘poor’ by Advisors.
- Physiological instability was noted in 322/444 (73%) of patients who subsequently had a cardiac arrest.
- Advisors considered that warning signs for cardiac arrest were present in 344/462 (75%) of cases. These warning signs were recognised poorly, acted on infrequently, and escalated to more senior doctors infrequently.
- There was no evidence of escalation to more senior staff in patients who had multiple reviews.
- Advisors considered that the cardiac arrest was predictable in 289/454 (64%) and potentially avoidable in 156/413 (38%) of cases.
- The Advisors reported problems during the resuscitation attempt in 91/526 cases (17%). Of these, 36/91 were associated with airway management.
- Survival to discharge after in-hospital cardiac arrest was 14.6% (85/581).
- Only 9/165 (5.5%) patients who had an arrest in asystole survived to hospital discharge.
- Survival to discharge after a cardiac arrest at night was much lower than after a cardiac arrest during the day time (13/176; 7.4% v 44/218; 20.1%).
In the opinion of the treating clinicians, earlier treatment of the problem and better monitoring may have improved outcome:
Compare these findings with a smaller scale confidential enquiry into the care of patients who ended up in intensive care units, published exactly 14 years ago by McQuillan et al2:
“The main causes of suboptimal care were failure of organisation, lack of knowledge, failure to appreciate clinical urgency, lack of supervision, and failure to seek advice.”
One of the co-authors of the McQuillan study, Professor Gary Smith , has spent years improving training in and awareness of the importance of recognition of critical illness, and pioneered the “ALERT” Course TM: Acute Life-threatening Emergencies, Recognition, and Treatment. Professor Smith provides commentary on the NCEPOD report and the slides are available here, including a reminder of the ‘Chain of Prevention’3.
It’s a shame these issues remain a problem but it is heartening to see NCEPOD tackle this important topic and provide recommendations that UK hospitals will have to act upon. It is further credit to the vision of Pete McQuillan, Gary Smith and their colleague Bruce Taylor (another co-author of the 1998 confidential inquiry). These guys opened my eyes to the world of critical care and trained me for 18 months on their ICU, which remains a beacon site for critical care expertise and training. Without their inspiration, I may not have ended up in emergency medicine-critical care and I doubt very much that Resus.ME would exist.
1. Cardiac Arrest Procedures: Time to Intervene? (2012)
National Confidential Enquiry into Patient Outcome and Death (NCEPOD)
2. Confidential inquiry into quality of care before admission to intensive care
BMJ 1998 Jun 20;316(7148):1853-8 Free Full Text
[EXPAND Click to read abstract]
OBJECTIVE: To examine the prevalence, nature, causes, and consequences of suboptimal care before admission to intensive care units, and to suggest possible solutions.
DESIGN: Prospective confidential inquiry on the basis of structured interviews and questionnaires.
SETTING: A large district general hospital and a teaching hospital.
SUBJECTS: A cohort of 100 consecutive adult emergency admissions, 50 in each centre.
MAIN OUTCOME MEASURES: Opinions of two external assessors on quality of care especially recognition, investigation, monitoring, and management of abnormalities of airway, breathing, and circulation, and oxygen therapy and monitoring.
RESULTS: Assessors agreed that 20 patients were well managed (group 1) and 54 patients received suboptimal care (group 2). Assessors disagreed on quality of management of 26 patients (group 3). The casemix and severity of illness, defined by the acute physiology and chronic health evaluation (APACHE II) score, were similar between centres and the three groups. In groups 1, 2, and 3 intensive care mortalities were 5 (25%), 26 (48%), and 6 (23%) respectively (P=0.04) (group 1 versus group 2, P=0.07). Hospital mortalities were 7 (35%), 30 (56%), and 8 (31%) (P=0.07) and standardised hospital mortality ratios (95% confidence intervals) were 1.23 (0.49 to 2.54), 1.4 (0.94 to 2.0), and 1.26 (0.54 to 2.48) respectively. Admission to intensive care was considered late in 37 (69%) patients in group 2. Overall, a minimum of 4.5% and a maximum of 41% of admissions were considered potentially avoidable. Suboptimal care contributed to morbidity or mortality in most instances. The main causes of suboptimal care were failure of organisation, lack of knowledge, failure to appreciate clinical urgency, lack of supervision, and failure to seek advice.
CONCLUSIONS: The management of airway, breathing, and circulation, and oxygen therapy and monitoring in severely ill patients before admissionto intensive care units may frequently be suboptimal. Major consequences may include increased morbidity and mortality and requirement forintensive care. Possible solutions include improved teaching, establishment of medical emergency teams, and widespread debate on the structure and process of acute care.
[/EXPAND]
3. In-hospital cardiac arrest: is it time for an in-hospital ‘chain of prevention’?
Resuscitation. 2010 Sep;81(9):1209-11
[EXPAND Click to read abstract]
The ‘chain of survival’ has been a useful tool for improving the understanding of, and the quality of the response to, cardiac arrest for many years. In the 2005 European Resuscitation Council Guidelines the importance of recognising critical illness and preventing cardiac arrest was highlighted by their inclusion as the first link in a new four-ring ‘chain of survival’. However, recognising critical illness and preventing cardiac arrest are complex tasks, each requiring the presence of several essential steps to ensure clinical success. This article proposes the adoption of an additional chain for in-hospital settings–a ‘chain of prevention’–to assist hospitals in structuring their care processes to prevent and detect patient deterioration and cardiac arrest. The five rings of the chain represent ‘staff education’, ‘monitoring’, ‘recognition’, the ‘call for help’ and the ‘response’. It is believed that a ‘chain of prevention’ has the potential to be understood well by hospital clinical staff of all grades, disciplines and specialties, patients, and their families and friends. The chain provides a structure for research to identify the importance of each of the various components of rapid response systems.
[/EXPAND]
Is diastolic worse than systolic dysfunction in sepsis?
 Septic myocardial dysfunction is a well recognised contributor to shock in sepsis but for many of us we assume this to be gross systolic impairment. Interestingly a recent study highlights that patients with severe sepsis and septic shock frequently have diastolic dysfunction1. They found that diastolic dysfunction was the strongest independent predictor of early mortality, even after adjusting for the APACHE-II score and other predictors of mortality.
Septic myocardial dysfunction is a well recognised contributor to shock in sepsis but for many of us we assume this to be gross systolic impairment. Interestingly a recent study highlights that patients with severe sepsis and septic shock frequently have diastolic dysfunction1. They found that diastolic dysfunction was the strongest independent predictor of early mortality, even after adjusting for the APACHE-II score and other predictors of mortality.
In this study, 9.1% of severe sepsis/septic shock patients had isolated systolic dysfunction, 14.1% had combined systolic and diastolic dysfunction, and 38% had isolated diastolic dysfunction.
Importantly, the authors point out that although diastolic dysfunction is associated with age, hypertension, diabetes mellitus, and ischaemic heart disease, diastolic dysfunction is a stronger independent predictor of mortality than age and the other co-morbidities. However, a limitation of the study acknowledged by the authors is that it did not include follow-up echocardiography examinations, so we do not know whether sepsis was responsible for a transient diastolic dysfunction or whether the observed diastolic dysfunction was a pre-existing condition.
Both troponin and NT-ProBNP elevations also predicted mortality.
Want to know how to measure diastolic dysfunction? These authors measured mitral annular early-diastolic peak velocity, or the e’-wave (called ‘e prime’). It is a way of seeing how fast myocardial tissue relaxes in diastole, and if its peak velocity is slow (in this case < 8cm/s) there is diastolic dysfunction. We measure speed using Doppler, and in this case we’re looking at the speed of heart tissue (as opposed to the blood cells within the heart chambers) so we do ‘Tissue Doppler Imaging’, or TDI. You need an echo machine with pulsed-wave Doppler, and you need to be able to get an apical view. This is explained really nicely here2 but if you don’t have the time or the echopassion to read a whole article on TDI watch this one minute video (BY emergency physicians FOR emergency physicians!) on diastology, where TDI measurement of e’ is shown from 45 seconds into the video.
For reference, there is some more detail on diastolic function measurements at the Echobasics site.
If you think you can cope with any more of this level of awesomeness and want these geniuses to talk to you from your smartphone in the ED then get the free One Minute Ultrasound app for Android or Apple devices.
AIMS: Systolic dysfunction in septic shock is well recognized and, paradoxically, predicts better outcome. In contrast, diastolic dysfunction is often ignored and its role in determining early mortality from sepsis has not been adequately investigated.
METHODS AND RESULTS: A cohort of 262 intensive care unit patients with severe sepsis or septic shock underwent two echocardiography examinations early in the course of their disease. All clinical, laboratory, and survival data were prospectively collected. Ninety-five (36%) patients died in the hospital. Reduced mitral annular e’-wave was the strongest predictor of mortality, even after adjusting for the APACHE-II score, low urine output, low left ventricular stroke volume index, and lowest oxygen saturation, the other independent predictors of mortality (Cox’s proportional hazards: Wald = 21.5, 16.3, 9.91, 7.0 and 6.6, P< 0.0001, <0.0001, 0.002, 0.008, and 0.010, respectively). Patients with systolic dysfunction only (left ventricular ejection fraction ≤50%), diastolic dysfunction only (e’-wave <8 cm/s), or combined systolic and diastolic dysfunction (9.1, 40.4, and 14.1% of the patients, respectively) had higher mortality than those with no diastolic or systolic dysfunction (hazard ratio = 2.9, 6.0, 6.2, P= 0.035, <0.0001, <0.0001, respectively) and had significantly higher serum levels of high-sensitivity troponin-T and N-terminal pro-B-type natriuretic peptide (NT-proBNP). High-sensitivity troponin-T was only minimally elevated, whereas serum levels of NT-proBNP were markedly elevated [median (inter-quartile range): 0.07 (0.02-0.17) ng/mL and 5762 (1001-15 962) pg/mL, respectively], though both predicted mortality even after adjusting for highest creatinine levels (Wald = 5.8, 21.4 and 2.3, P= 0.015, <0.001 and 0.13).
CONCLUSION: Diastolic dysfunction is common and is a major predictor of mortality in severe sepsis and septic shock.
1. Diastolic dysfunction and mortality in severe sepsis and septic shock
Eur Heart J. 2012 Apr;33(7):895-903
2. A clinician’s guide to tissue Doppler imaging
Circulation. 2006 Mar 14;113(10):e396-8 Free Full Text
Thrombolytic Therapy in Unstable Patients with PE
Most of us would give strong consideration to giving thrombolytics to patients with massive pulmonary embolism (PE), which is in keeping with many guidelines. Some physicians remain reluctant to do so, often citing the lack of good evidence. It is true that large scale RCTs have not been done in this population. The authors of this recent retrospective study state:
There are no definitive trials that prove the value of thrombolytic therapy in unstable patients with pulmonary embolism. It is extremely remote that a randomized controlled trial will be performed in the future. We therefore analyzed the database of the Nationwide Inpatient Sample to test the hypothesis that thrombolytic therapy reduces case fatality rate in unstable patients with acute pulmonary embolism.
They demonstrate a striking difference in mortality when thrombolysis is given to unstable patients with PE, which is further reduced with the addition of a vena cava filter. ‘Unstable’ was defined as having a listed code for shock or ventilator dependence.
Associated comorbid conditions were more often present in those who did not receive thrombolytic therapy than in those who did. However in their discussion the authors add:
Although unstable patients who received thrombolytic therapy had fewer comorbid conditions than those who did not, this would not explain the difference in case fatality rate because unstable patients with a primary diagnosis of pulmonary embolism and none of the comorbid conditions…also showed a lower case fatality rate with thrombolytic therapy. Therefore, differences in comorbid conditions in this group were eliminated as a possible cause of the lower case fatality rate in unstable patients who received thrombolytic therapy.
They round off their conclusion with:
Despite the marked reduction of case fatality rate with thrombolytic therapy in unstable patients, only 30% of unstable patients received it, and the proportion receiving thrombolytic therapy is diminishing. On the basis of these data, thrombolytic therapy in combination with a vena cava filter in unstable patients with acute pulmonary embolism seems indicated.
Many thanks to Dr Daniel Horner for highlighting this paper.
BACKGROUND: Data are sparse and inconsistent regarding whether thrombolytic therapy reduces case fatality rate in unstable patients with acute pulmonary embolism. We tested the hypothesis that thrombolytic therapy reduces case fatality rate in such patients.
METHODS: In-hospital all-cause case fatality rate according to treatment was determined in unstable patients with pulmonary embolism who were discharged from short-stay hospitals throughout the United States from 1999 to 2008 by using data from the Nationwide Inpatient Sample. Unstable patients were in shock or ventilator dependent.
RESULTS: Among unstable patients with pulmonary embolism, 21,390 of 72,230 (30%) received thrombolytic therapy. In-hospital all-cause case fatality rate in unstable patients with thrombolytic therapy was 3105 of 21,390 (15%) versus 23,820 of 50,840 (47%) without thrombolytic therapy (P< .0001). All-cause case fatality rate in unstable patients with thrombolytic therapy plus a vena cava filter was 505 of 6630 (7.6%) versus 4260 of 12,850 (33%) with a filter alone (P<.0001). Case fatality rate attributable to pulmonary embolism in unstable patients was 820 of 9810 (8.4%) with thrombolytic therapy versus 1080 of 2600 (42%) with no thrombolytic therapy (P<.0001). Case fatality rate attributable to pulmonary embolism in unstable patients with thrombolytic therapy plus vena cava filter was 70 of 2590 (2.7%) versus 160 of 600 (27%) with a filter alone (P<.0001).
CONCLUSION: In-hospital all-cause case fatality rate and case fatality rate attributable to pulmonary embolism in unstable patients was lower in those who received thrombolytic therapy. Thrombolytic therapy resulted in a lower case fatality rate than using vena cava filters alone, and the combination resulted in an even lower case fatality rate. Thrombolytic therapy in combination with a vena cava filter in unstable patients with acute pulmonary embolism seems indicated.
Thrombolytic Therapy in Unstable Patients with Acute Pulmonary Embolism: Saves Lives but Underused
Am J Med. 2012 May;125(5):465-70
Hypotonic Versus Isotonic Fluids After Surgery for Children
Kids in hospital with injury, infection or other illness, and those undergoing the physiological stress of surgery, produce (appropriately) elevated antidiuretic hormone levels which contribute to the risk of hyponatraemia by impairing free water excretion in the kidney.
Deaths have occurred on general paediatric and surgery wards when fluid regimens containing low concentrations of sodium (classically 0.18% or 0.225% NaCl) have resulted in hyponatraemia in children without adequate electrolyte monitoring, leading some bodies to recommend at least 0.45% NaCl solutions for maintenance fluid therapy in children.
However two recent studies1,2 on postoperative children show an increased risk of hyponatraemia even with 0.45% saline, when compared with 0.9% saline or Hartmann’s solution (Hartmann’s is similar – almost identical – to Ringer’s lactate).
I like the fact that paediatricians used Hartmann’s in one of these studies1. I have worked with several paediatricians who never use Hartmann’s, either from lack of experience or because of concern about its lactate content (not appreciating the lactate is metabolised by the liver to bicarbonate).
This is ironic, since Alexis Hartmann (1898–1964) was a paediatrician.
Want more fluid therapy irony? The ‘balanced salt solution’ used by Brits and Australasians is Hartmann’s solution – named after an American. The one used by Americans is Lactated Ringer’s solution – named after the British physician Sydney Ringer (1834-1910).
Medical history enthusiasts can read more about Hartmann and Ringer here.
1. A randomised controlled trial of Hartmann’s solution versus half normal saline in postoperative paediatric spinal instrumentation and craniotomy patients.
Arch Dis Child. 2012 Jun;97(6):491-6
[EXPAND Click to read abstract]
OBJECTIVE: To compare the difference in plasma sodium at 16-18 h following major surgery in children who were prescribed either Hartmann’s and 5% dextrose or 0.45% saline and 5% dextrose.
DESIGN: A prospective, randomised, open label study.
SETTING: The paediatric intensive care unit (650 admissions per annum) in a tertiary children’s hospital in Brisbane, Australia.
PATIENTS: The study group comprised 82 children undergoing spinal instrumentation, craniotomy for brain tumour resection, or cranial vault remodelling.
INTERVENTIONS: Patients received either Hartmann’s and 5% dextrose at full maintenance rate or 0.45% saline and 5% dextrose at two-thirds maintenance rate.
MAIN OUTCOMES MEASURES: Primary outcome measure: plasma sodium at 16-18 h postoperatively; secondary outcome measure: number of fluid boluses administered.
RESULTS: Mean postoperative plasma sodium levels of children receiving 0.45% saline and 5% dextrose were 1.4 mmol/l (95% CI 0.4 to 2.5) lower than those receiving Hartmann’s and 5% dextrose (p=0.008). In the 0.45% saline group, seven patients (18%) became hyponatraemic (Na <135 mmol/l) at 16-18 h postoperatively; in the Hartmann’s group no patient became hyponatraemic (p=0.01). No child in either fluid group became hypernatraemic.
CONCLUSIONS: The postoperative fall in plasma sodium was smaller in children who received Hartmann’s and 5% dextrose compared to those who received 0.45% saline and 5% dextrose. It is suggested that Hartmann’s and 5% dextrose should be administered at full maintenance rate postoperatively to children who have undergone major surgery in preference to hypotonic fluids.
[/EXPAND]
2. Hypotonic versus isotonic maintenance fluids after surgery for children: a randomized controlled trial
Pediatrics. 2011 Nov;128(5):857-66.
[EXPAND Click to read abstract]
OBJECTIVE: The objective of this randomized controlled trial was to evaluate the risk of hyponatremia following administration of a isotonic (0.9% saline) compared to a hypotonic (0.45% saline) parenteral maintenance solution (PMS) for 48 hours to postoperative pediatric patients.
METHODS: Surgical patients 6 months to 16 years of age with an expected postoperative stay of >24 hours were eligible. Patients with an uncorrected baseline plasma sodium level abnormality, hemodynamic instability, chronic diuretic use, previous enrollment, and those for whom either hypotonic PMS or isotonic PMS was considered contraindicated or necessary, were excluded. A fully blinded randomized controlled trial was performed. The primary outcome was acute hyponatremia. Secondary outcomes included severe hyponatremia, hypernatremia, adverse events attributable to acute plasma sodium level changes, and antidiuretic hormone levels.
RESULTS: A total of 258 patients were enrolled and assigned randomly to receive hypotonic PMS (N = 130) or isotonic PMS (N = 128). Baseline characteristics were similar for the 2 groups. Hypotonic PMS significantly increased the risk of hyponatremia, compared with isotonic PMS (40.8% vs 22.7%; relative risk: 1.82 [95% confidence interval: 1.21-2.74]; P = .004). Admission to the pediatric critical care unit was not an independent risk factor for the development of hyponatremia. Isotonic PMS did not increase the risk of hypernatremia (relative risk: 1.30 [95% confidence interval: 0.30-5.59]; P = .722). Antidiuretic hormone levels and adverse events were not significantly different between the groups.
CONCLUSION: Hypotonic Versus Isotonic Maintenance Fluids After Surgery for Children: A Randomized Controlled Trial.
[/EXPAND]
T waves in V1-V3 were not associated with badness
This long term follow up study showed that T-wave inversions in right precordial leads are not associated with adverse outcome.
Background-: T-wave inversion in right precordial leads V1 to V3 is a relatively common finding in a 12-lead ECG of children and adolescents and is infrequently found also in healthy adults. However, this ECG pattern can also be the first presentation of arrhythmogenic right ventricular cardiomyopathy. The prevalence and prognostic significance of T-wave inversions in the middle-aged general population are not well known.
Methods and Results-: We evaluated 12-lead ECGs of 10 899 Finnish middle-aged subjects (52% men, mean age 44+/-8.5 years) recorded between 1966 and 1972 for the presence of inverted T waves and followed the subjects for 30+/-11 years. Primary end points were all-cause mortality, cardiac mortality, and arrhythmic death. T-wave inversions in right precordial leads V1 to V3 were present in 54 (0.5%) of the subjects. In addition, 76 (0.7%) of the subjects had inverted T waves present only in leads other than V1 to V3. Right precordial T-wave inversions did not predict increased mortality (not significant for all end points). However, inverted T waves in leads other than V1 to V3 were associated with an increased risk of cardiac and arrhythmic death (P<0.001 for both).
Conclusions-: T-wave inversions in right precordial leads are relatively rare in the general population, and are not associated with adverse outcome. Increased mortality risk associated with inverted T waves in other leads may reflect the presence of an underlying structural heart disease.
Prevalence and prognostic significance of T-wave inversions in right precordial leads of a 12-lead electrocardiogram in the middle-aged subjects
Circulation. 2012 May 29;125(21):2572-7
Nonshockable arrest survival improves with uninterrupted compressions
A study of nonshockable out of hospital cardiac arrest survival showed significant improvement in short- and long-term survival and neurological outcome after implementation of a protocol consistent with CPR guidelines that prioritised chest compressions. These improvements were especially evident among arrests attributable to a cardiac cause, although there was no evidence of harm among arrests attributable to a noncardiac cause.
This was not a randomised trial so unrecognised factors may have contributed to the improved outcome in addition to the change in CPR protocol. However, it is interesting as it provides up to date survival rates from a large population sample: Non shockable out of hospital cardiac arrests achieve return of spontaneous circulation in 34%, 6.8% are discharged from hospital (5.1% with a favourable neurological outcome), and 4.9% survived one year.
The breakdown between PEA and asystole is of course telling, and unsurprising, with 12.8% versus 1.1% being discharged with a favourable neurological outcome, respectively. I would imagine then that some of the PEA patients had beating hearts with hypotension extreme enough to cause pulselessness (pseudo-electromechanical dissociation) – clinically a ‘cardiac arrest’ but really nothing of the sort, and the reason we use cardiac ultrasound to prognosticate.
BACKGROUND: Out-of-hospital cardiac arrest (OHCA) claims millions of lives worldwide each year. OHCA survival from shockable arrhythmias (ventricular fibrillation/ tachycardia) improved in several communities after implementation of American Heart Association resuscitation guidelines that eliminated “stacked” shocks and emphasized chest compressions. “Nonshockable” rhythms are now the predominant presentation of OHCA; the benefit of such treatments on nonshockable rhythms is uncertain.
METHODS AND RESULTS: We studied 3960 patients with nontraumatic OHCA from nonshockable initial rhythms treated by prehospital providers in King County, Washington, over a 10-year period. Outcomes during a 5-year intervention period after adoption of new resuscitation guidelines were compared with the previous 5-year historical control period. The primary outcome was 1-year survival. Patient demographics and resuscitation characteristics were similar between the control (n=1774) and intervention (n=2186) groups, among whom 471 of 1774 patients (27%) versus 742 of 2186 patients (34%), respectively, achieved return of spontaneous circulation; 82 (4.6%) versus 149 (6.8%) were discharged from hospital, 60 (3.4%) versus 112 (5.1%) with favorable neurological outcome; 73 (4.1%) versus 135 (6.2%) survived 1 month; and 48 (2.7%) versus 106 patients (4.9%) survived 1 year (all P≤0.005). After adjustment for potential confounders, the intervention period was associated with an improved odds of 1.50 (95% confidence interval, 1.29-1.74) for return of spontaneous circulation, 1.53 (95% confidence interval, 1.14-2.05) for hospital survival, 1.56 (95% confidence interval, 1.11-2.18) for favorable neurological status, 1.54 (95% confidence interval, 1.14-2.10) for 1-month survival, and 1.85 (95% confidence interval, 1.29-2.66) for 1-year survival.
CONCLUSION: Outcomes from OHCA resulting from nonshockable rhythms, although poor by comparison with shockable rhythm presentations, improved significantly after implementation of resuscitation guideline changes, suggesting their potential to benefit all presentations of OHCA.
Impact of changes in resuscitation practice on survival and neurological outcome after out-of-hospital cardiac arrest resulting from nonshockable arrhythmia
Circulation. 2012 Apr 10;125(14):1787-94
Not a pin cushion
This is the daughter of my friend. Avery is only seven months old and has survived a critical illness and is thankfully now fully recovered. Her Dad has nothing but praise for the medical and nursing staff who cared for her. But one thing could have been better. Avery endured multiple attempts at vascular access without ultrasound guidance.
If you were her parent, and you were an emergency physician with galaxy-class expertise in emergency ultrasound, how would you react? Complaints? Incident forms? Outrage?
How about education? For free. Accompanied by lavish praise for the experts who treated Avery and made her better.
Avery’s Dad is ultrasound podcaster and gentleman Dr Matt Dawson. He is offering FREE ultrasound training to anyone who wants to improve their vascular access skills.
Are there nurses, physicians, or technicians in your ED or ICU that could improve their care with this training? Please consider sending them for this training. To register for the course, and to read Avery’s full story, go to notapincushion.com.
And if you’re already comfortable with ultrasound-guided vascular access, then visit the site anyway, as there is some education here for all of us: how to turn a gut-wrenchingly distressing experience into something positive that will benefit countless others. I am thoroughly inspired.
Best wishes to an amazing family.
Cliff
Laryngospasm after Ketamine
A case is reported in Prehospital Emergency Care1 in which an agitated patient (due to mania and alcohol intoxication) received 5 mg/kg (500 mg) of ketamine intramuscularly by an EMS crew which dissociated him within a few minutes. He subsequently developed episodes of laryngospasm in the emergency department which were unrelieved by head tilt, chin lift and simple airway adjuncts but responded to bag-mask ventilation (BMV). The patient was intubated because the laryngospasm recurred, although it had again responded to BMV.
The authors make the point that because of the response of laryngospasm to simple manoeuvres, and because in the prehospital environment a patient will not be left without an EMS provider present, ‘restricting ketamine to EMS units capable of rapid-sequence intubation therefore seems unnecessary.‘
This is one for EMS directors to consider seriously. Personally, I think practicing prehospital care without access to ketamine is like having a hand tied behind my back. Ketamine opens up a world of possibilities in controlling combative patients, optimising scene safety, providing sedation for painful procedures including extrication, and enabling severe pain to be controlled definitively.
I’ve been using ketamine regularly for prehospital analgesia and emergency department procedural sedation in both adults and kids for more than a decade. I’ve seen significant laryngospasm 5 times (twice in kids). On one of those occasions, a 3 year old child desaturated to around 50% twice during two episodes of laryngospasm. We weren’t slow to pick it up – that was just her showing us how quickly kids can desaturate which continued while we went through a stepwise approach until BMV resolved it. It was however an eye opener for the registrar (senior resident) assisting me, who became extremely respectful of ketamine after that. Our ED sedation policy (that I wrote) required that suxamethonium was ready and available and that an appropriate dose had been calculated before anyone got ketamine. Paralysis may extremely rarely be required, but when it’s needed you need to be ready.
Laryngospasm is rare but most regular prescribers of ketamine will have seen it; the literature says it occurs in about 1-2% of sedations, although anecdotally I think it’s a bit less frequent. Importantly for those weighing the risks of allowing non-RSI competent prescribers, the requirement for intubation is exceptionally rare (2 of 11,589 reported cases in one review). Anyone interested should read this excellent review of ketamine-related adverse effects provided by Chris Nickson at Life in The Fast Lane. Chris reminds us of the Larson manouevre, which is digital pressure in the notch behind and below the ear, described by Larson2 as follows:
The technique involves placing the middle finger of each hand in what I term the laryngospasm notch. This notch is behind the lobule of the pinna of each ear. It is bounded anteriorly by the ascending ramus of the mandible adjacent to the condyle, posteriorly by the mastoid process of the temporal bone, and cephalad by the base of the skull. The therapist presses very firmly inward toward the base of the skull with both fingers, while at the same time lifting the mandible at a right angle to the plane of the body (i.e., forward displacement of the mandible or “jaw thrust”). Properly performed, it will convert laryngospasm within one or two breaths to laryngeal stridor and in another few breaths to unobstructed respirations.
I use this point most often to provide painful stimuli when assessing GCS in a patient, particular those I think may be feigning unconsciousness (I’ve done this for a number of years since learning how painful it can be when I was shown it by a jujitsu instructor). Dr Larson says he was taught the technique by Dr Guadagni, so perhaps we should be calling it the ‘Guadagni manouevre’. The lack of published evidence has led to some appropriate skepticism3, but as it can be combined with a jaw thrust it needn’t delay more aggressive interventions should they become necessary, it may work, and it’s likely to be harmless.
I presented the following suggested algorithm for management of laryngospasm during ketamine procedural sedation at a regional emergency medicine ‘Fellows Forum’ meeting in November 2007 in the UK. Since many paediatric procedural sedations were done using intramuscular (im) ketamine, it gives guidance based on whether or not vascular access has been obtained:
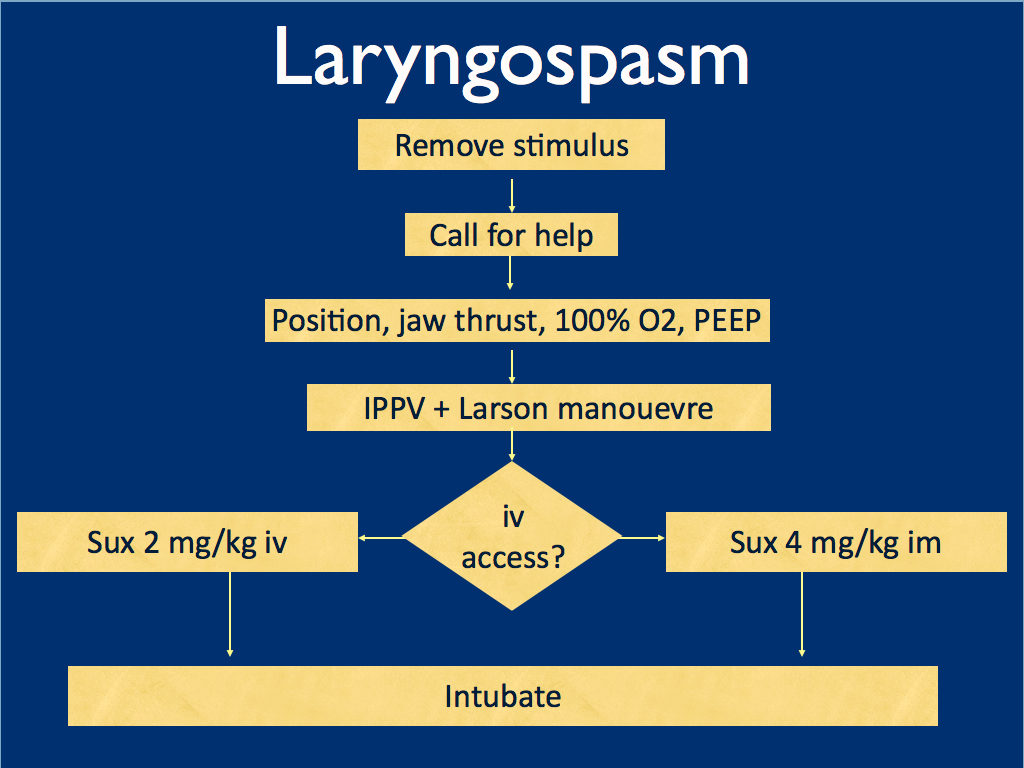
Some things I considered were:
-
- Neuromuscular blockade (NMB) isn’t always necessary – laryngospasm may be managed with other sedatives such as propofol. However, titrating further sedatives in a desaturating child in my view is inferior to definitive airway management and laryngeal relaxation with suxamethonium and a tube.
-
- Laryngospasm may be managed with much smaller doses of suxamethonium than are required for intubation – as little as 0.1 mg/kg may be effective. However, I think once we go down the NMB route we’re committed to intubation and therefore we should use a dose guaranteed to be effective in achieving intubating conditions.
-
- In the child without vascular access, I considered intraosseous and intralingual sux. However, intramuscular suxamethonium is likely to have a relaxant effect on the laryngeal muscles within 30-45 seconds, which has to be compared with time taken to insert and confirm intraosseous needle placement. I do not think the traditionally recommended intralingual injection has any role to play in modern airway management.
- At the time I wrote this most paediatric resuscitation bays in my area in the United Kingdom had breathing circuits capable of delivering PEEP – usually the Ayr’s T-Piece (specifically the Mapleson F system), which is why PEEP was included early in in the algorithm prior to BMV.
ABSTRACT An advanced life support emergency medical services (EMS) unit was dispatched with law enforcement to a report of a male patient with a possible overdose and psychiatric emergency. Police restrained the patient and cleared EMS into the scene. The patient was identified as having excited delirium, and ketamine was administered intramuscularly. Sedation was achieved and the patient was transported to the closest hospital. While in the emergency department, the patient developed laryngospasm and hypoxia. The airway obstruction was overcome with bag–valve–mask ventilation. Several minutes later, a second episode of laryngospasm occurred, which again responded to positive-pressure ventilation. At this point the airway was secured with an endotracheal tube. The patient was uneventfully extubated several hours later. This is the first report of laryngospam and hypoxia associated with prehospital administration of intramuscular ketamine to a patient with excited delirium.
1. Laryngospasm and Hypoxia After Intramuscular Administration of Ketamine to a Patient in Excited Delirium
Prehosp Emerg Care. 2012 Jul;16(3):412-4
2. Laryngospasm-The best treatment
Anesthesiology 1998; 89:1293-4
3. Management of Laryngospasm
http://www.respond2articles.com/ANA/forums/thread/1096.aspx









